1 Which one is a chemical change-rusting of iron or melting of iron?
Ans: Rusting of iron.
2 Name and state the law which is kept in mind while we balance a chemical equation
Ans: Law of conservation of mass: Mass can neither be created nor be destroyed during a chemical reaction.
3 State one basic difference between a physical change and a chemical change.
Ans: In a physical change, no new substance is formed. In a chemical change, a new substance is forme.
4 What happens when quicklime is added to water?
Ans: Quicklime reacts with water vigorously to produce slaked lime and a large amount of heat is liberated.
CaO (s) + H2O → Ca (OH)2 + Heat
Quick Lime slaked Lime
5 What happens when ZnCO3 is heated in the absence of air? Give the relevant equation
Ans: ZnO (s) and CO (g) are formed.
![]()
6. Is burning of a candle, a physical change or a chemical change?
Ans: Both, chemical change and physical change.
7. Write the chemical equation for reactions that take place when lead nitrate and potassium iodide solutions are mixed
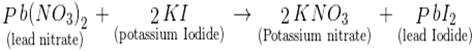
8. Write a balanced chemical equation:
![]()
Ans: Balanced equation is ![]()
9. Which one is the chemical change – Fermentation of fruit juice or diluting fruit juice?
Ans: Fermentation of fruit juice.
10. What is meant by the skeletal equation?
Ans: An unbalanced equation is called a skeletal equation.
11. What is observed when carbon dioxide gas is passed through lime water?
(i) For a short duration?
(ii) For long duration?
Also, write the chemical equations for the reaction involved.
Ans: (i) Lime water turns milky due to the formation of CaCO3, which is insoluble in water
![]()
(ii) A clear solution is obtained due to the formation of calcium bicarbonate.
Ca (HCO3) which is soluble in water
![]()
12. Why do we store silver chloride in dark colored bottles? Explain in brief.
Ans: Silver chloride on exposure to sunlight undergoes photolytic decomposition.
![]()
Therefore it is stored in dark colored bottles.
13. Define a combination reaction. Give one example of a combination reaction which is also exothermic.
Ans: A combination reaction is said to have occurred when two or more than two substances combine to form a single substance.
![]()
14: What happen chemically when quicklime is added to water filled in a bucket?
Ans. Quick lime react with water to form slaked lime and produces lot of heat and hissing sound.
CaO + H2O → Ca(OH)2 + heat + hissing sound

