6.2 TEXT QUESTIONS:
1: List conditions under which combustion can take place.
Ans. Conditions under which combustion can take place are as follows:
i) Fuel is required.
ii) Air (oxygen) is necessary.
iii) Ignition temperature is required.
2 :Fill in the blanks:
(a) Burning of wood and coal causes pollution of air.
(b) A liquid fuel, used in homes is Liquified Petroleum Gas (LPG).
(c) Fuel must be heated to its ignition temperature before it starts burning.
(d) Fire produced by oil cannot be controlled by water.
3: Explain how the use of CNG in automobiles has reduced pollution in our cities.
Ans. CNG produces very small amounts of harmful products like Sulphur dioxide, Oxides of nitrogen, Oxides of sulphur etc. CNG is a cleaner fuel. So the pollution is reduced
4:Compare LPG and wood as fuels.
| Properties | LPG | Wood |
| 1. Energy/unit | Produces more energy | Produces less energy |
| 2. Pollution | Less pollution | More pollution |
| 3. Residue | Leave no residue | Leave ash as residue |
| 4. Storage/Transport | Easy to store & transport | Needs more space to store |
5: Give reasons:
i). Water is not used to control fires involving electrical equipment.
Ans. Water is not used to control fires involving electrical equipment because it may conduct electricity and harm those trying to douse the fire
ii). LPG is a better domestic fuel than wood.
Ans. LPG is a good fuel as compared to wood as it readily available and it is cheap. It burns easily in air at a moderate rate and it produces a large amount of heat. It does not leave behind any undesirable substances.
iii). Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not.
Ans. Paper by itself catches fire easily whereas a piece of paper wrapped around an aluminium pipe does not because the ignition temperature of paper is low as compare to the paper wrapped around an aluminium pipe.
6: Make a labelled diagram of a candle flame.
Ans.
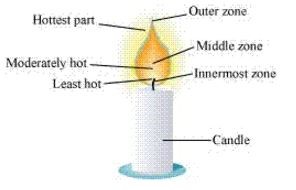
7: Name the unit in which the calorific value of a fuel is expressed.
Ans. The calorific value of a fuel is expressed in a unit called kilojoules per kg (kJ/kg).
8: Explain how CO2 is able to control fires.
Ans. CO2, being heavier than oxygen, covers the fire like a blanket. Since the contact between the fuel and oxygen is cut off, the fire is controlled.
9: It is difficult to burn a heap of green leaves but dry leaves catch fire easily. Explain.
Ans. The ignition temperature of green leaves is higher than that of the dry leaves, so dry leaves catch fire easily. For everything to burn it has to reach its ignition temperature.
10: Which zone of a flame does a goldsmith use for melting gold and silver and why.
Ans. Goldsmith uses the outermost zone of the flame for melting gold and silver because it is the hottest zone (complete combustion) of the flame.
11: In an experiment 4.5 kg of a fuel was completely burnt. The heat produced was measured to be 180,000 kJ. Calculate the calorific value of the fuel.
Ans. Calorific Value = Heat produced (ink)/Mass of fuel (in kg) = 180,000 kj/4.5 kg = 1800000/4.5 = 40000 kj/kg
12: Can the process of rusting be called combustion. Discuss.
Ans: No, rusting is a very slow process as compared to combustion and the heat evolved in combustion is much more than rusting. Rusting can take place at room temperature but combustion needs an ignition temperature.
13: Abida and Ramesh were doing an experiment in which water was to be heated in a beaker. Abida kept the beaker near the wick in the yellow part of the candle flame. Ramesh kept the beaker in the outermost part of the flame. Whose water will get heated in a shorter time.
Ans. The outermost part of the flame is the hottest one, so Ramesh’s water will get heated in a shorter time

