1. What is meant by a functional group in an organic compound? Name the functional group present in (i) CH3CH2OH (ii) CH3COOH
Ans: (a) Functional group is an atom or group of atoms or reactive part of compound, which determines the chemical properties of compounds.
(i) —OH (Alcohol)
(ii) —COOH (Carboxylic acid)
2. Give reasons for the following observations:
(a) The element carbon forms a very large number of compounds.
(b) Air holes of a gas burner have to be adjusted when the heated vessels get blackened by the flame.
Ans: (a) Carbon forms large number of compounds since carbon is small in size and can form stable covalent bonds (catenation) and it shows tetravalency.
(b) Air holes of gas burner are made open (adjusted) so that air can pass through, which is needed for complete combustion, so that heated vessels do not get blackened.
3. What is Ethanoic acid? Write the formula of the functional group present in this acid.
How does Ethanoic acid react with sodium carbonate?
Ans: CH3COOH is ethanoic acid. –COOH is the formula of the functional group present in Ethanoic acid.
Ethanoic acid reacts with sodium carbonate to give brisk effervescence due to carbon dioxide gas are formed.
4. State two characteristic features of carbon which when put together give rise to large
number of carbon compounds.
Ans. (i) Catenation (ii) Tetravalency of carbon
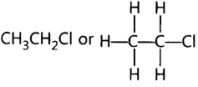
5. Write the structural formula of Chloroethane.
Ans: Chloroethane = CH3CH2Cl
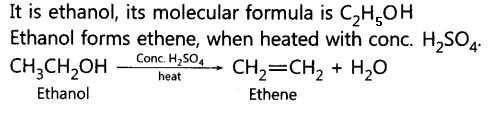
6. Write the name and molecular formula of an organic compound having its name suffixed with ‘-ol and having two carbon atoms in the molecule. With the help of a
balanced chemical equation indicate what happens when it is heated with excess of concentrated.H2S04.
Ans:
7. Write the names and molecular formula of two organic compounds having functional r group suffix as ‘-oic acid’. With the help of a balanced chemical equation and explain what happens when any one of them reacts with sodium hydroxide.
Ans:
8. What is a homologous series? Which two of the following organic compounds belong to the same homologous?
CH3, C2H6, C2H6O, C2H6O2, CH4O
Ans: Homologous series is a series of organic compounds that have the same functional group and similar chemical properties. Each member of this series differs by –CH2- in its molecular formula and 14 u in its molecular mass. (C2H5OH) and (CH3OH) belong to the same homologous series.
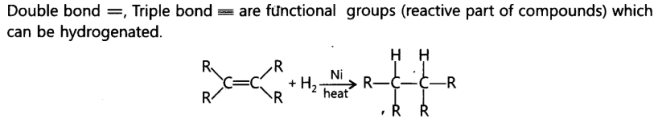
9. Name the functional group of organic compounds that can be hydrogenated. Name any one natural source of organic compounds that are hydrogenated.
Ans:Vegetable oils are hydrogenated to form vegetable ghee. Plants are natural sources of vegetable oils that can be hydrogenated.

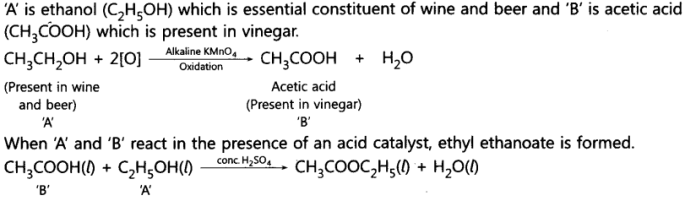
10. An organic compound ‘A’ is an essential constituent of wine and beer. Oxidation of ‘A’ yields an organic acid ‘B’ which is present in vinegar. Name the compounds ‘A’ and ‘B’ and write their structural formula. What happens when ‘A’ and ‘B’ react in the presence of an acid catalyst? Write the chemical equation for the reaction.
Ans:
11. What is Ethanol? State its two properties. What happens when it is heated with excess of concentrated H2SO4 at 443 K . What role does concentrated H2SO4 play in this reaction? Write chemical equation of the reaction involved and the structural formula of the main product formed.
Ans:
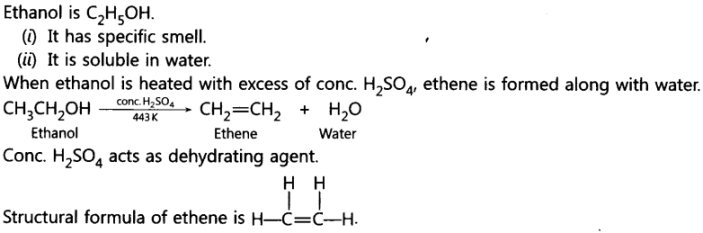
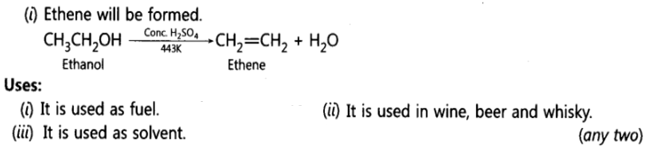
12. With the help of balanced chemical equations explain what happens when ethanol is heated with excess concentrated sulphuric acid at 443 K. Mention any three uses of ethanol.
Ans:
13. How many covalent bonds are there in a molecule of Ethane (C2H6)?
Ans: There are 7 covalent bonds in a molecule of Ethane.
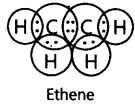
14. Write the electron dot structure of Ethene molecule (C2H4).
Ans:
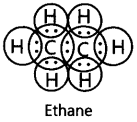
15. Write the electron dot structure of ethane molecule (C2H6).
Ans:
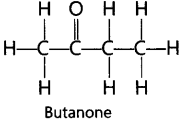
16. Draw the structure of Butanone molecule, CH3COC2H5.
Ans:
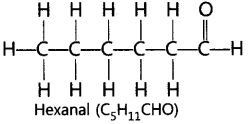
17. Draw the structure of the hexanal molecule, C5H11CHO.
Ans:
18. Butanone is a four carbon per molecule compound. Name the functional group present in it.
Ans: Ketone
19. Name the following compound:
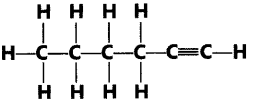
Ans: 1-Hexyne is IUPAC name of the compound.

